Details of the Drug
General Information of Drug (ID: DMVY0BU)
| Drug Name |
Apaziquone
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
EOquin; 114560-48-4; Apaziquonum; NOR-701; EO 9 (pharmaceutical); EO-9; Apaziquone [USAN:INN]; NSC-382459; Apaziquonum [INN-Latin]; E 09; NSC 382459; UNII-H464ZO600O; E-85/053; E-09; EO9; NSC 382456; H464ZO600O; 5-(Azridin-1-yl)-3-(hydroxymethyl)-2-((1E)-3-hydroxyprop-1-enyl)-methyl-1H-indole-4,7-dione; (E)-5-(1-Azirinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-1H-indole-4,7-dione; E09; 1H-Indole-4,7-dione, 5-(1-aziridinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-, (E)-; Neoquin; Qapzola; Apaziquonum; EO 9; Eoquin (TN); Apaziquone (USAN/INN); E-85/050; 3-hydroxymethyl-5-aziridinyl-1-methyl-2-(1H-indole-4,7-dione)prop-beta-en-alpha-ol; 5-(aziridin-1-yl)-3-(hydroxymethyl)-2-[(E)-3-hydroxyprop-1-enyl]-1-methylindole-4,7-dione; Apaziquone/EOquin
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
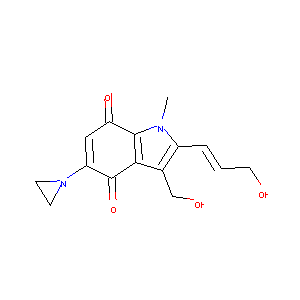 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 288.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.5 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
